Introduction to Chlorine Residual Testing.
Chlorine is a powerful disinfectant and is widely used by water utilities. It destroys the membrane of many microorganisms and kills them. When sufficient chlorine is added to water, some of it reacts with microbes and other organic matter, disinfecting the water. Some chlorine reacts with inorganic compounds to form combined chlorine. Chlorine that remains after meeting the demand for disinfection and formation of combined chlorine, is called free residual chlorine or simply residual chlorine (RC).
How does drinking water reach our homes? Most consumers having municipal water connections would know that drinking water reaches our homes through pipes. This seemingly simple service requires elaborate infrastructure, operations, maintenance and quality control arrangements to supply safe drinking water. Metro and municipal water utilities usually source water from various surface water bodies, such as dams & reservoirs built across rivers, irrigation canal systems, direct-draw from rivers, fresh water lakes, etc. Where surface water sources are unavailable or inadequate, and high yielding groundwater aquifers are available, cities and towns may rely wholly or partially on borewells.
Figure 1: Basic Schema of Municipal Water Utilities
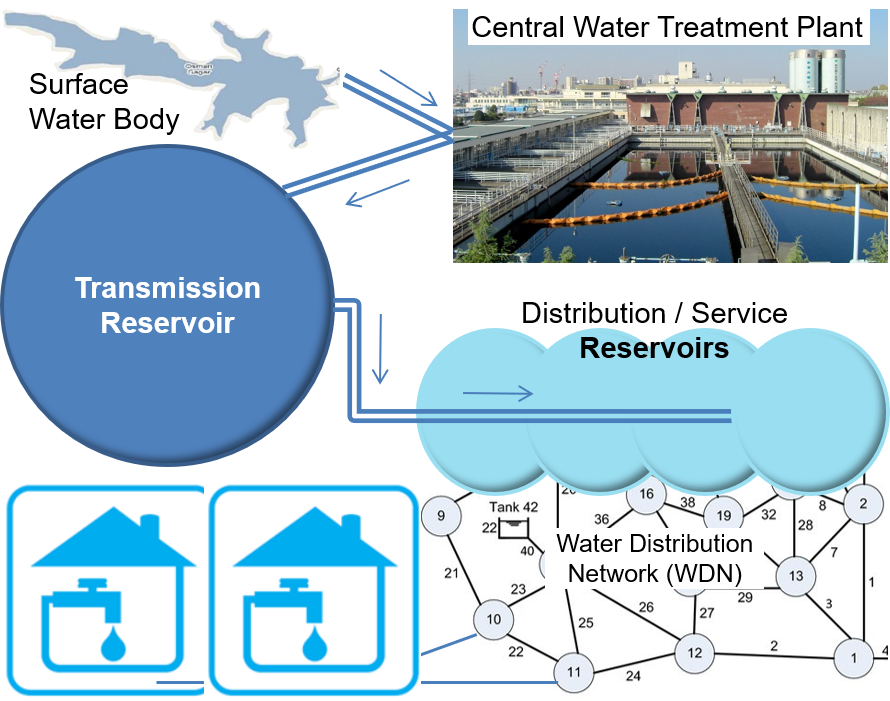
Despite, various water safety measures, the quality of water from each source would vary inherently on account of differences in exposure to minerals and contaminants. Central treatment plants (popularly called filter beds) clarify the water, perform quality checks and balance chemical parameters if required. Finally, the Central Treatment Plant disinfects (kill or neutralise microbial contaminants, if any) by chlorination. After removing microbial contamination, Central Treatment Plants release water to transmission reservoirs and Service Reservoirs with enough residual chlorine to prevent recontamination during the course of transmission and storage. In other words, residual chlorine in treated water is a kind of security protection against any microbial contamination, on the way. Levels of residual chlorine gradually reduce as treated water travels over the distribution network because of reactions with natural organic matter and pipe-wall materials. Thus, the residual chlorine level reduces, by the time treated water reach Service Reservoirs. Hence, booster chlorination is done at Service Reservoirs to raise the level of residual chlorine, before supply to households through distribution networks.
Figure 2: Some Service Reservoir - Booster Chlorination Plants.

The presence of chlorine residual in drinking water indicates that: 1) a sufficient amount of chlorine was added initially to the water to inactivate the bacteria and some viruses that cause diarrheal disease; and, 2) the water is protected from recontamination during storage & transmission. The presence of free residual chlorine in drinking water is correlated with the absence of disease-causing organisms, and thus is a measure of the potability of water. When chlorine cannot be detected in a municipal tap water, this indicates that either contamination has entered somewhere in the pipe lines from service reservoir to the tap or enough chlorine was not added at the Service Reservoir.
Three types of chlorine residual can be measured. These are;
- Free residual chlorine: which kills microorganisms most effectively. Free residual chlorine is often called simply as “Residual Chlorine” or “RC” or “Free Chlorine” or “Chlorine Residual”. All these terms are synonymous. In the water treatment utilities and municipal water departments the abbreviation “RC” invariably means “Residual Chlorine”. In India, most municipal water utilities use chlorine gas for disinfection, which when added in sufficient quantity leaves free residual chlorine (FRC or more commonly abbreviated as RC).
- Combined chlorine: When free chlorine reacts with various chemicals in water, combined chlorine is formed. Some types of combined chlorine do kill microorganisms but too slowly. As mentioned above, RC is most effective in killing microorganisms that may enter into intermediate storage and the distribution system. Combined chlorine is also formed in swimming pools, where its measurement is important to know quality of pool water.
- Total chlorine: Free Residual Chlorine + Combined Chlorine = Total Chlorine.
Testing for Free Residual Chlorine and Total Chlorine is comparatively easier than testing for combined chlorine. Thus, when we want to know the level of combined chlorine in water, we test for Total Chlorine and subtract the measured level of Free RC. If we know that the level of combined chlorine is insignificant, tests for free chlorine is not available, Total Chlorine test results can be used as approximate indicator of free residual chlorine.
Free Chlorine in water is unstable and the amount of chlorine in water may go down quickly, especially in warm climate like that of Hyderabad. Sunlight makes free chlorine to disappear quickly. Shaking of water in a container will also make Free Chlorine disappear soon. Hence, Chlorine testing should be carried out on-site.
Guidelines for Residual Chlorine in Drinking Water Supplies:
The World Health Organization (WHO) recommends that there should always be a minimum of 0.5mg/L RC at all points in a piped supply, particularly when there is risk of cholera. In areas where there is little risk of a cholera outbreak, there should be a chlorine residual of 0.2 to 0.5 mg/L at all points. Too much chlorine in water gives rise to a bleaching powder smell. Hence, the WHO specifies a maximum permissible limit of 5.0mg/L residual chlorine.
The Indian standard for drinking water quality (IS10500, 2012) specifies a general minimum of 0.2mg/L tested at the consumer-end (households). When protection against viral infection is required, the minimum residual chlorine level at the consumer end should be 0.5mg/L. The maximum permissible level of residual chlorine is set at 1.0mg/L by IS10500 for aesthetic reasons. Considering that Hyderabad does sporadically experience waterborne viral disease outbreaks such as hepatitis (jaundice), the targeted consumer-end minimum residual chlorine level should be 0.5mg/L.
Testing Methods:
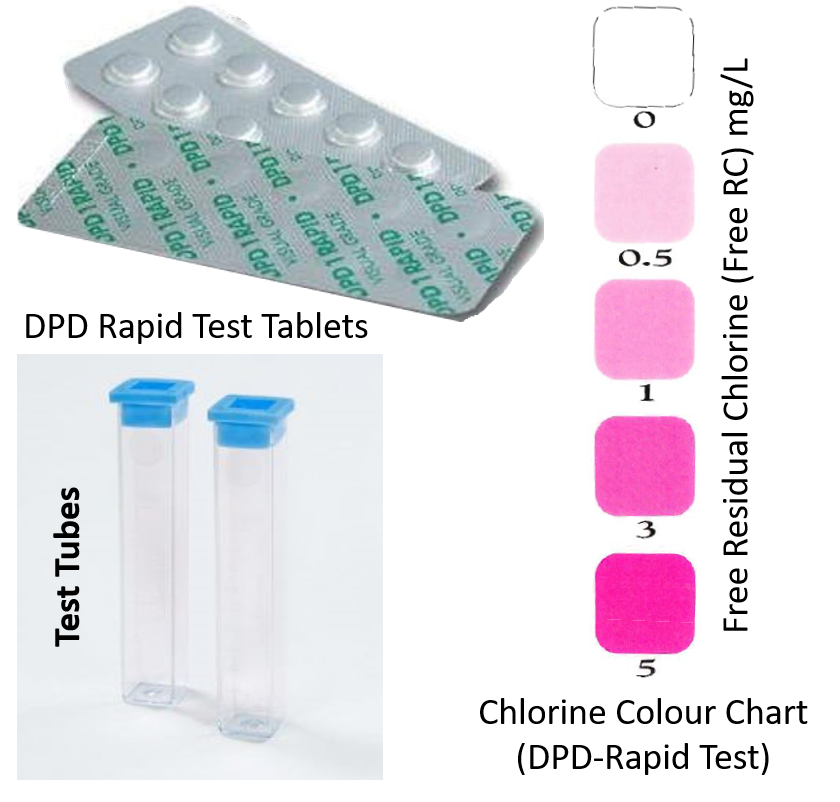
The quickest and simplest method of testing for chlorine residual is the DPD (diethyl paraphenylene diamine) indicator test, using DPD #1 Rapid, visual grade tablets, a 5-ml test tube and a laminated colour comparator chart. First, rinse the test tube with the water to be tested. Then, refill the tube upto 5 ml mark. Position the PD 1R tablet blister strip on top of the test tube and press from other side to release a tablet directly from the blister pack, into the tube. Wait till the tablet dissolves completely. Read results immediately after the tablet is dossolved. If RC is present, colour of test-sample turns to a shade of red depending on the level of free chlorine. Measure strength of colour against standard colours on a chart to determine the chlorine concentration. The stronger the colour, the higher the concentration of chlorine in the water.
Figure 3: Contents of a DPD Rapid Test Kit
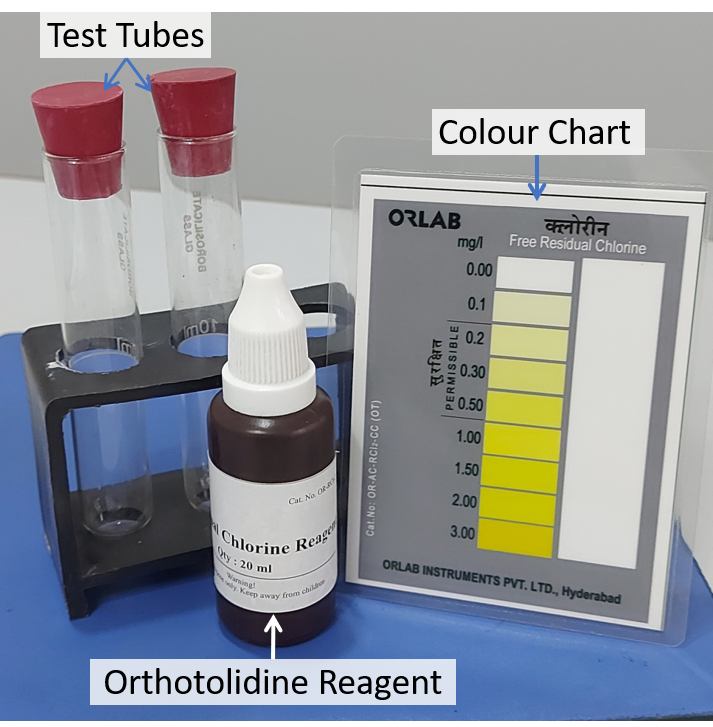
When Orthotolidine-arsenite (OTA) is added to a test-sample, it reacts with free residual chlorine in water producing a yellow-coloured compound. Intensity of yellow colour is proportional to the level free residual chlorine. Hence, comparison of colour developed in test-sample with a calibrated colour chart gives test result. Repeated and long-term exposure to Orthotolidine increases the risk of bladder cancer. Hence, the World Health Organization (WHO), recommends to prefer the safer DPD method. If the OTA method is chosen, Water Quality Investigators can minimise their risk by avoiding direct contact with the Orthotolidine reagent.
Figure 4: Contents of a Orthotolidine Rapid Test Kit
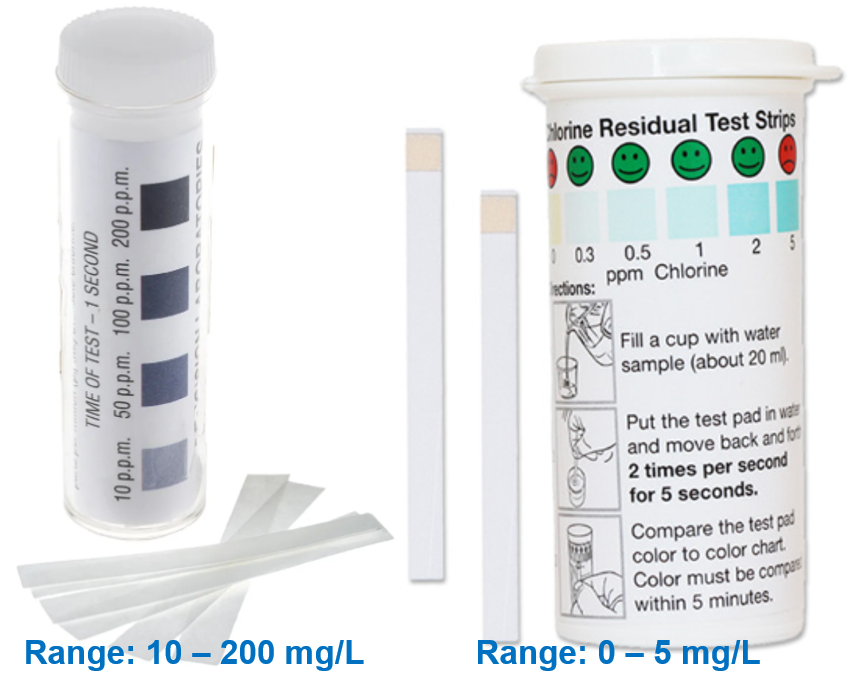
The starch-iodide test uses a potassium iodide starch test strip designed to detect low levels of chlorine in the range of 0 to 5mg/L or 0 to 10mg/L. The test-strip, when dipped in water for about 5 seconds, develops blue to purple colour depending on the level of total chlorine the test-sample. Note that this method measures total chlorine. Since, free residual chlorine is important to assure water safety, the starch-iodide method is usually not recommended. However, in cases where we know that formation of combined chlorine is limited and DPD test is not available, the starch-iodide method can give some idea of the residual chlorine level.
Figure 5: Starch-Iodide Test Strips
References:
- WHO. 2016. Chlorine Testing Fact Sheet. Geneva: World Health Organization (WHO); Fact Sheet: 2.31.
- WHO. 2013. Measuring chlorine levels in water supplies. Geneva: World Health Organization (WHO); 2013 Jul; Technical Notes Drinking-Water, Sanitation and Hygiene in Emergencies. https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/environmental-health-in-emergencies/technical-notes-on-wash-in-emergencies
- WHO. 1996. Chlorine in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Geneva: World Health Organization (WHO).
- IS 10500. Indian Standard Drinking Water Specification. Second Revision. New Delhi: Bureau of Indian Standard (BIS); 2012 May. https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf
- IARC. ortho-Toluidine. in: IARC. Chemical Agents and Related Occupations. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans Volume 100F. Lyon, France: International Agency for Research on Cancer (IARC); 2012; pp. 93-100. https://publications.iarc.fr/123



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
