Groundwater Potability Tests (GPT)
Scope:

Physical & Sensory Parameters:
Colour (Appearance & True Colour Units), Odour, Turbidity,
pH, Electrical Conductivity (EC) &
Total Dissolved Solids (TDS) by gravimetry (TDSG).

Chemical Parameters:
Alkalinity, Hardness, Calcium (Ca) & Magnesium (Mg); Ammoniacal-Nitrogen (NH3-N), Nitrite (NO2) & Nitrate (NO3); Fluoride, Boron,
Chloride, Sulfate, Iron (Fe) & Manganese (Mn).

Bacteriological Parameters:
Detection of Total coliforms & E. coli in 100 ml; by
Membrane Filtration & Chromogenic agar plating (MFC),
Or Enzyme Substrate Autoanalysis (ESA).
Total parameters: 6 + 13 + 2 = 21
Rationale:
There are many aspects to drinking water quality contributing to potability. Water that is free of faecal contamination is relatively safe. But pathogen free water may not be palatable. For example, high mineral content in groundwater may affect palatability. The palatability of drinking water has been rated by panels of tasters in relation to total dissolved solids (TDS). Water with TDS higher than 1200 mg/L is generally unpalatable. Certain components of TDS, such as chlorides, sulfates, magnesium, calcium, and carbonates, affect corrosion or encrustation in water-distribution systems. High TDS levels (>500 mg/litre) result in excessive scaling in water pipes, water heaters, boilers, and household appliances such as kettles and steam irons (WHO, 2003).
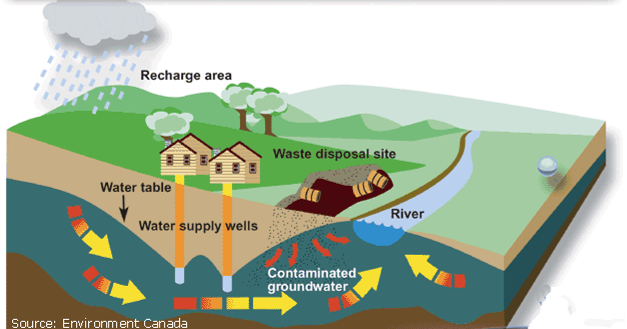
As a source, groundwater has certain inherent advantages, such as local availability, no evaporation loss, on-demand availability. Rocks act as a natural filter for water accumulated in deep aquifers. Deep aquifers are usually a good source of clean water. Groundwater is likely to be free of pathogenic bacteria and can generally be used without further treatment. However, possibility of faecal contamination exit. Aquifer recharge zones may extend far and wide. Contamination of an aquifer from any part of its recharge zone can pollute whole of the aquifer.
Improper installation and inadequate maintenance can affect quality of ground water. Ideally, systematic well development process should be followed, while commissioning new borewells. Well development is a treatment of a well to establish the maximum rate of usable water yield without sand content beyond permissible limit for turbidity (IS: 11189, 1985). Similarly, grouting and sealing of casing pipe is required to prevent contaminated surface water ingress into a borewell. Open wells ought to be covered, have pumping arrangement, and the perimeter well drained. Water quality tests at regular intervals help monitor integrity of borewells /open wells and fitness for intended purpose.
In GPT we have packaged a comprehensive set of Organoleptic & physical, chemical and bacteriological parameters, to check for wholesomeness, buffering capacity and suitability for various domestic uses. Fluoride is included as fluorosis is endemic in some areas around Hyderabad. TDS value would be a point estimate based on actual weighment (gravimetry). Groundwater often contains higher levels of iron and manganese compared with surface waters, that are mostly the primary source for municipal water supplies. Formation of coloured layer on top of groundwater stored in open tanks, is usually due to iron in water. Manganese can form coatings on water pipes that may later slough off as a black precipitate. GPT includes iron and manganese in addition to all parameters included in CPT. This is a cost-effective means to assess the quality of borewell well water.

The Indian standard specification for drinking water quality (IS 10500, 2012) lists 64 parameters including, 3 bacteriological, 6 organoleptic & physical, 24 general chemical parameters, 11 toxic substances, 18 pesticide residues and 2 radioactive substances. Inclusion in the standard specification does not mean that all 64 quality parameters have to be tested all the time. Choice of parameters for monitoring and verification of quality is to be based on reliability of the source, variability of parameter values, prevalence of attributable risk, consumer concerns and clues leading to suspicious contamination. The groundwater potability tests covers all organoleptic & physical parameters, except for taste, which would be known to the consumer. In addition, appearance (naked eye observation of unfiltered sample for colour) and electrical conductivity are included. Differences between appearance and true colour helps in interpretation of results. Electrical conductivity is commonly used to characterise salinity of surface waters and is also helpful to cross check the TDS estimate. All 3 of the bacteriological parameters are included. And 11 out of 24 general chemical parameters listed in IS10500 of 2012 are included. In addition, phenolphthalein alkalinity and nitrite are included. Explicit testing and reporting of phenolphthalein alkalinity help in computation of alkalinity components, namely; carbonates, bicarbonates and hydroxides, that may help interpretation for certain purposes. Nitrite is included in the WHO Guidelines and is an indicator of recent or continuing environmental contamination. GPT does not include any of the toxic substances, pesticide residues or radioactive substances listed in IS10500. Where contamination with pesticides and any other toxic substances is suspected, additional tests should be considered.
Although, GPT is primarily designed with drinking and domestic use of groundwater, in view, the test package can be used for other sources and uses as well. GPT can be used to assess suitability of groundwater for animal feed and poultry. Groundwater is sometimes used in fish ponds. GPT would be relevant wherever groundwater is used as a source of raw water. Bottled water and reverse osmosis (RO) plants often use groundwater as feed water. GPT can be used to assess the need for RO plants, design of bottled water processing plants, sizing of RO plant components, and to monitor performance of RO plants.
Go TopSample - Collection, Storage & Transportation:
Indian Standard (IS) 17614 (Part 1) of 2021 provides guidance on the design of sampling programs and sampling techniques. IS 17614 (Part 5) of 2021 provides guidance on sampling of drinking water from treatment works and piped distribution systems. IS1622:1981 provides sampling guidance for bacteriological tests. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather all that you need for collection of water sample:
One litre sample collected in a clean and dry clear or amber colour polypropylene bottle (CBWS/ABWS) is required for physical and chemical tests. About 250 ml of water sample collected in a sterile bottle with thiosulfate (SBT) is required for bacteriological tests. You need a pair of sample collection bottles (1xCBWS + 1xSBT), two black or dark colour polythene bags (small garbage bag will do) to minimise exposure of samples to sunlight, ice packs to keep the sample bottles cool during transport and a carry bag for convenient transport.
Both CBWS and SBT are available from the IHS Laboratory. If it is not feasible for you to collect the specified sample collection bottles from the laboratory and you must collect samples, freshly emptied packaged drinking water bottles or new PET bottles may be used, in that order. If only one freshly emptied packaged water bottle is available, then use the same to collect bacteriological sample. Do not use empty beverage bottles such as ThumsUp, Maaza, Sprite. Nutrients and other residues in such bottles may promote growth of bacteria and bias test results.
Step-2: Identify sampling point and time:
For borewell with handpump, the handpump delivery pipe is the obvious choice. In case of borewells with submersible or external pumps, look for a tap on the delivery pipe closest to the borewell head. In the absence of a convenient tap from the delivery pipe, collect sample from the delivery pipe termination inside the storage tank as borewell water falls into the tank. If this is not feasible, collect samples from taps connected to the storage tank. If taps and delivery pipes are not available and the well water container has a narrow mouth, then pouring water into the sample collection bottle would be an option. In case of open well, use pump delivery pipe. In the absence of pump use water drawing arrangement for the well and draw water from inside the well using a clean bucket or vessel.
Step-3: Collect sample:
- Wash both your hands with soap and water, wipe with a clean towel and let it dry. Request an assistant to wash his/her hands and standby.
- Label the sample collection bottles and place it within easy reach, but do not open at this stage. Have ice packs ready.
- Flush the delivery pipe by letting water out for, say 2-3 minutes. Do not touch the flow from delivery pipe. Ask the assistant to operate handpump or draw water from open well and pour for sample collection by you.
- Collect the sample for physical and chemical analysis first; followed by collection of bacteriological-sample.
- e. Hold the bottle in one hand and open and hold the cap in the other hand avoiding to touch inner side of the cap. Place opened bottle mouth under the delivery pipe or spout avoiding direct contact. As the bottle is about to be full, quickly remove the bottle away from water stream and replace cap tightly. Wipe outside of bottle dry with a clean and dry tissue or cloth.
- Place each bottle inside separate dark colour bags, tie ice packs around each of them and place it in a carry bag for transport to laboratory.
Step-4: Transport to laboratory:
Transport samples to laboratory as soon as possible, preferably within six hours. If you have multiple errands in the same trip, plan to first deposit sample at the laboratory & continue with others.
Step-5 Store sample, if required:
If immediate transport is not feasible, store the sample inside the regular chamber (not the freezer compartment) of a refrigerator until you are ready to transport it to the Laboratory, and definitely within 24 hours from the time of collection.
Go TopInformation About Source, Context, Intended Use & Concerns:
Details regarding location, age of the borewell, its depth, pump capacity, usually helps proper interpretation of test results. GPS coordinates and photos of the of the borewell location would be useful. Provide as much detail as you can about the sampling point, and how the source borewell is connected to the sampling point. Mention about intended use of the water, the reason why you are ordering the test, as well as doubts and concerns, if any. These information help in interpretation of test results. Occasionally, the IHS Laboratory may contact you for clarifications and additional information about the source and its environment, to help interpretation of test results.
Test Method & Duration:
Physical and chemical characteristics of water sample are tested according appropriate parts of the IS3025 and/or American Public Health Association (APHA). For bacteriological analysis methods specified in IS 1622 of 1981 are used. Report will be available in 3 to 5 days, depending on duration of bacteriological analysis and gathering of additional information, if any is required.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- APHA. 2017. Standard Methods for the Examination of Water and Wastewater, 23rd Edition. Washington, DC: American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation. https://www.standardmethods.org/
- IS 1622: 1981. Indian Standard Methods of Sampling and Microbiological Examination of Water. New Delhi: Bureau of Indian Standard (BIS); Indian Standard, IS1622 - 1981, Reaffirmed 1996, Amended 2003. https://law.resource.org/pub/in/bis/S02/is.1622.1981.pdf
- IS 3025 Part 1: 1987. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater. Part 1 Sampling. New Delhi: Bureau of Indian Standard (BIS); Indian Standard. Reaffirmed 2003. https://law.resource.org/pub/in/bis/S02/is.3025.01.1987.pdf
- IS 3025 Relevant Parts: 1983. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater. Parts, 4, 10, 21,46 etc. New Delhi: Bureau of Indian Standard (BIS); Indian Standard. https://www.services.bis.gov.in/php/BIS_2.0/bisconnect/knowyourstandards/indian_standards/isdetails
- IS 10500. Indian Standard Drinking Water Specification. Second Revision. New Delhi: Bureau of Indian Standard (BIS); 2012 May. https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf
- IS 2800 of 1991 Part 1, Code of Practice for Construction and Testing of Tube wells / Borewells. Part 1 Construction, Second Revision, 1991 reaffirmed in 2001.
- IS:11189-1985 Methods of Tube well development, New Delhi: Bureau of Indian Standard (BIS); 1985 Reaffirmed 1999; https://law.resource.org/pub/in/bis/S08/is.11189.1985.pdf
- WHO. 2003. Total dissolved solids in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Geneva: World Health Organization (WHO); 2003. https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/chemical-hazards-in-drinking-water/total-dissolved-solids
- WHO. 2011. Guidelines for drinking-water quality. Fourth Edition. Geneva: 2011. https://www.who.int/publications/i/item/9789241549950
