City-water Potability Tests (CPT)
Scope:

Physical & Sensory Parameters:
Colour (Appearance & True Colour Units), Odour, Turbidity, pH, Electrical Conductivity (EC) & Total Dissolved Solids (TDS) by gravimetry (TDSG).

Chemical Parameters:
Alkalinity, Total Water Hardness (TWH), Calcium (Ca) & Magnesium (Mg); Ammoniacal-Nitrogen (NH3-N), Nitrites (NO2) & Nitrates (NO3); Fluoride, Boron, Chloride & Sulfate.

Bacteriological Parameters:
Detection of Total coliforms & E. coli in 100 ml by Membrane Filtration (MF) Or Enzyme Substrate (ES) Method.
Total parameters: 6 + 11 + 2 = 19
Rationale:
There are many aspects to drinking water quality contributing to potability. Water that is free of faecal contamination is relatively safe. But pathogen free water may not quench thirst. For example, distilled water is safe to drink but would taste flat or metallic! Low-mineral content water may taste poorly and has also been reported to be less thirst quenching. Poor taste and thirst-quenching characteristics may affect the amount of water consumed or cause persons to seek other, possibly less satisfactory water sources (Kozisek 2005). On the other hand, high mineral content would also affect palatability. Water with TDS higher than 1200 mg/L is generally unpalatable (WHO, 2003). Physical and sensory characteristics such as colour, odour, and turbidity are aesthetically important and may also indicate the possibility of some contamination that is not readily detected by routine tests. Exposure to water and its constituents can occur through ingestion, contact and aerosol inhalation. Hence, WHO considers that drinking-water should also be suitable for washing, showering and domestic food preparation.
Metro and municipal water supplies are prima-facie reliable sources of potable water. Raw water is mostly from surface water bodies, as in case of Hyderabad. Public water utilities monitor a wide range of quality parameters and adjust their treatment processes to deliver potable water with acceptable physical, chemical and microbial characteristics. However, structural and operational deficiencies in transmission, storage or local distribution network may alter the quality of water dispatched by central treatment plants. Consumer-end surveillance of water quality improves accountability, identifies local vulnerabilities, and strengthens overall water safety. Structural and operational deficiencies of in-campus, residential-cluster, apartment complex water storage distribution system, as well as vulnerabilities in domestic storage and plumbing may introduce contamination. For example, spreading tree roots may crack sump-wall and facilitate development of a siphon between domestic sewage lines and sump water. Bird droppings into open overhead tanks, can introduce faecal contamination. Point-of-use water purifiers may fail. Hence, periodical testing of direct municipal supplies, water stored in sumps, overhead tanks, tap water that is required to check for wholesomeness of water and integrity of domestic water storages and plumbing.

In CPT we have packaged a comprehensive set of Organoleptic & physical, chemical and bacteriological parameters, to check for wholesomeness, buffering capacity and suitability for home appliances, of water from metro or municipal supplies. Fluoride is included as fluorosis is endemic in some areas around Hyderabad. TDS value would be a point estimate based on actual weighment (gravimetry). The coliform tests leading up to identification of E. coli is essential for determining whether a health risk exists. This is a cost-effective means to verify the quality of metro or municipal supplies and integrity of consumer-end water infrastructure.
The Indian standard specification for drinking water quality (IS10500, 2012) lists 64 parameters including, 2 bacteriological parameters, 6 organoleptic & physical parameters, 24 general chemical parameters, 12 toxic substances, 18 pesticide residues and 2 radioactive substances. Inclusion in the standard specification does not mean that all 64 quality parameters have to be tested all the time. Choice of parameters for monitoring and verification of quality is to be based on reliability of the source, variability of parameter values, prevalence of attributable risk, consumer concerns and clues leading to suspicious contamination. The city-water potability tests covers all organoleptic & physical parameters, except for taste, which would be known to the consumer. In addition, appearance (naked eye observation of unfiltered sample for colour) and electrical conductivity are included. Differences between appearance and true colour units helps in interpretation of results. Electrical conductivity is commonly used to characterise salinity of surface waters and is also helpful to cross check the TDS estimate. Both of the bacteriological parameters are included. And 10 out of 24 general chemical parameters listed in IS10500 of 2012 are included. Nitrite is included, in addition, because it is an indicator of recent or continuing environmental contamination. Nitrite is also included WHO guidelines for drinking water quality. CPT does not include any of the toxic substances, pesticide residues or radioactive substances listed in IS10500. Where contamination with pesticides and any other toxic substances is suspected, additional tests should be considered.
Although, CPT is primarily designed with municipal supplies in view, the test package may be used for other sources such as bottled and packaged water, RO water, bore wells, if parameters in the scope of this test are considered adequate and the client can interpret test results in the specific circumstances of each case.
Sample - Collection, Storage & Transportation:
Indian Standard (IS) 17614 (Part 1) of 2021 provides guidance on the design of sampling programs and sampling techniques. IS 17614 (Part 5) of 2021 provides guidance on sampling of drinking water from treatment works and piped distribution systems. IS1622:1981 provides sampling guidance for bacteriological tests. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather all that you need for collection of water sample:
One litre sample collected in a clean and dry clear or amber colour polypropylene bottle (CBWS/ABWS) is required for physical and chemical tests. About 250 ml of water sample collected in a sterile bottle with thiosulfate for drinking / fresh water (SBTD) is required for bacteriological tests. You need a pair of sample collection bottles (1 × CBWS + 1 × SBTD), two black or dark colour polythene bags (small garbage bag will do) to minimise exposure of samples to sunlight, ice packs to keep the sample bottles cool during transport and a carry bag for convenient transport.
Both CBWS and SBTD are available from the IHS Laboratory. If it is not feasible for you to collect the specified sample collection bottles from the laboratory and you must collect samples, freshly emptied packaged drinking water bottles or new PET bottles may be used, in that order. If only one freshly emptied packaged water bottle is available, then use the same to collect bacteriological sample. Do not use empty beverage bottles such as ThumsUp, Maaza, Sprite. Nutrients and other residues in such bottles may promote growth of bacteria and bias test results.
Step-2: Identify sampling point and time:
The best option is to identify a tap, spout or delivery pipe from which the sampling bottle can be filled. If there are many taps, identify a tap that is representative of the point-of-use. Remove tap-attachments, such as aerators and filters. For testing quality of municipal supply, select the direct municipal supply tap and choose a time during live municipal supply hours. To sample water from sumps, prefer pump-delivery either at the pump location or from inside the overhead tank (OHT) before water falls into the OHT. Similarly, prefer pump delivery pipes of borewells, and open wells. If taps and delivery pipes are not available and the water container has a narrow mouth, then pouring water into the sample collection bottle would be an option. But pouring option may not be available if the water storage container is so large that tipping it would not be feasible or it fixed as in case of a build overhead tank or underground sump. Thus, where tapping or pouring is not feasible a dip-sample would be the only option.
Step-3: Collect sample:
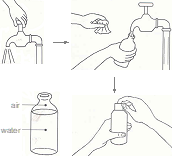
- Wash both your hands with soap and water, wipe with a clean towel and let it dry. Request an assistant to wash his/her hands and standby.
- Label the sample collection bottles and place it within easy reach, but do not open at this stage. Have ice packs ready.
- Then flush the tap, delivery pipe, spout by letting water out for, say 2-3 minutes. Do not touch the tap or water flowing out. In case of narrow mouth container, ask the assistant to tilt the container and pour water to flush the mouth. In case of dip sample, create a clear zone by carefully placing bottom of sample bottle on water surface and moving it in a cyclical to motion to disperse floating debris, if any.
- Collect the sample for physical and chemical analysis first; followed by collection of bacteriological-sample.
- Hold the sample collection bottle and wait for flushing of tap, delivery pipe, spout or narrow mouth is complete. Hold the bottle in one hand and open and hold the cap in the other hand avoiding to touch inner side of the cap. Place opened bottle mouth under the tap, delivery pipe, spout or mouth of the container avoiding direct contact with the tap. Ask the assistant to open the tap, delivery pipe control or tilt the container to pour water into the bottle. As the bottle is about to be full, quickly remove the bottle away from water stream and replace cap tightly. Wipe outside of bottle dry with a clean and dry tissue or cloth.
- Place each bottle inside separate dark colour bags, tie ice packs around each of them and place it in a carry bag for transport to laboratory.
Step-4: Transport to laboratory:
Transport the samples to laboratory as soon as possible, preferably within six hours. If you have multiple errands in the same trip, plan to first deposit sample the laboratory and then continue with other activities.
Step-5 Store sample, if required:
If immediate transport is not feasible, store the sample inside the regular chamber (not the freezer compartment) of a refrigerator until you are ready to transport it to the Laboratory, and definitely within 24 hours from the time of collection.
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the source of water, sampling point, activities & environment around the sampling point, details of how the sampling point is linked to the source, recent maintenance event (servicing of water purifier, cleaning of overhead tank, sump, repair and renovation of plumbing, etc.) if any. Mention about intended use of the water, the reason why you are ordering the test, as well as doubts and concerns, if any. These information help in interpretation of test results. Occasionally, the IHS Laboratory may contact you for clarifications and additional information about the source and its environment, to help interpretation of test results.
Test Method & Duration:
Organoleptic (sensory), physical and chemical characteristics of water sample are tested according as per appropriate parts of the IS3025 and/or American Public Health Association (APHA) Standard Methods. For bacteriological analysis methods specified in APHA Standard Methods or IS15185 of 2016 are used. Report will be available in 3 to 5 days, depending on duration of bacteriological analysis and gathering of additional information, if any is required.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- APHA. 2023. Standard Methods for the Examination of Water and Wastewater, 24th Edition. Washington, DC: American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation. https://www.standardmethods.org/
- IS 10500. Indian Standard Drinking Water Specification. Second Revision. New Delhi: Bureau of Indian Standard (BIS); 2012 May.
- Kozisek Frantisek. 2005. Health Risks from Drinking Demineralised Water. Ch-12 in: WHO. Nutrients in Drinking Water. Geneva: World Health Organization (WHO), 2005: 148-63. https://apps.who.int/iris/handle/10665/43403
- WHO. 2003. Total dissolved solids in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Geneva: World Health Organization (WHO); 2003. https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/chemical-hazards-in-drinking-water/total-dissolved-solids
- WHO. 2011. Guidelines for drinking-water quality. Fourth Edition. Geneva: 2011. https://www.who.int/publications/i/item/9789241549950
