Faecal Contamination Tests (FCT)
Scope:
The Faecal Contamination Coliform Tests (FCT) by IHS Laboratory includes two key parameters of the coliform test, namely; (a) detection of total coliforms and (b) presence or absence of Escherichia coli (E. coli). Detection of total coliforms indicates either inadequacy of disinfection or post-treatment recontamination with heterotrophs that may be harmless or pathogenic. Detection of E. coli, in addition to total coliforms, indicates faecal contamination.

Bacteriological Parameters:
Detection of Total coliforms & E. coli in 100 ml; by
Membrane Filtration & Chromogenic agar plating (MFC),
Or Enzyme Substrate Autoanalysis (ESA).
Total parameters: 2
Rationale:
Faecal contamination of water used for drinking, food processing, bathing and recreational activities such as swimming can cause several diseases including diarrhoea, dysentery, giardia, typhoid, jaundice, and polio. Microbial contamination of drinking water imposes immediate disease burden. Hence, bacteriological examination of water is necessary to determine potability and fitness for other uses that can expose people to water borne pathogens. The usual source of water borne pathogens is faecal contamination of human or animal origin.
Water borne pathogens include various microbial agent such as; Vibrio cholerae, Salmonella (typhoid, paratyphoid), Shigella dysenteriae, Hepatitis virus, etc. Direct identification of all water borne pathogens is complex, time consuming and expensive. Instead, looking for organisms that indicate faecal contamination provides a feasible and cost-effective alternative. Members of two bacteria groups, coliforms and fecal streptococci, are used as indicators of possible sewage contamination because they are commonly found in human and animal feces. Although they are generally not harmful themselves, they indicate the possible presence of disease-causing bacteria, viruses, and protozoans that also live in human and animal digestive systems.
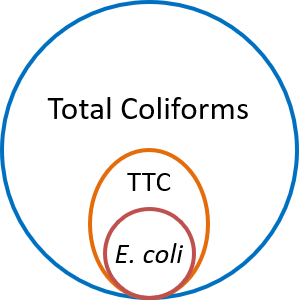
The coliform tests are based on culture of indicator bacteria to identify faecal contamination. Total coliforms are found everywhere. All members of the total coliform group can occur in human feces, but some can also be present in animal manure, soil, and submerged wood and in other places outside the human body. A subset of total coliforms that can survive and grow at relatively higher temperatures are called thermotolerant coliforms (TTC). Most thermotolerant coliforms inhabit the intestinal tract of man and animals in great abundance and hence are also known as faecal coliforms (FC). However, this group includes a genus such as Klebsiella, which is not necessarily of faecal origin. E. coli, which is a thermotolerant coliform is specific to fecal material from humans and other warm-blooded animals. Thus, while faecal coliforms are useful indicators of sewage contamination, presence of E. coli is a more specific indicator of faecal contamination. Hence detection of E. coli is a definite indicator of faecal contamination.
Coliform tests are cost-effective solution to rule out (or identify) faecal contamination of water. Water utilities all over the world rely on coliform tests to monitor water quality. The World Health Organization (WHO) recommends coliform bacteria as useful indicators for assurance of drinking water quality. Presence of E. coli is an indicator of contamination with human sewage. Total coliforms in the absence of E. coli) would be an indicator for cleanliness and integrity of distribution systems (WHO, 2011). The Indian Standard Drinking Water Specification requires that, in respect of all water intended for drinking, E. coli shall not be detectable in any 100 ml sample (IS10500, 2012).

Detection of E. coli is essential for determining whether a health risk exists. The same microbial parameters covered in this test (FCT) are included in various ‘Potability’ test packages such as BPT, CPT, CWF, HWF, HRO, BTLP, BPT, CPT, BWLP, and in some ‘Profile’ test packages such as ROP & GWSP. The ‘Potability’ and ‘Profile’ tests are necessary to benchmark the physical, chemical and microbial characteristic of water from a given source, treatment unit, storage, of point-of-use. Physical and chemical characteristics of water from the same source, treatment unit, storage or point-of-use do not usually change much, in the short-run. But insidious faecal contamination events can happen more often, due to various factors. The FCT is a cost-effective follow-up test solution for more frequent monitoring and to reassure safety of drinking water. However, appropriate ‘Potability” and/or ‘Profile’ tests should be repeated with regular periodicity, as appropriate to specific situation.
Sample - Collection, Storage & Transportation:
Follow methods of sampling specified in IS 1622 : 1981. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather all that you need for collection of water sample:
About 250 ml of water sample is required. You need a sterile sample collection bottle, a black or dark colour polythene bag (small garbage bag will do) to minimise exposure of sample to sunlight, ice packs to keep the sample bottle cool during transport and a carry bag for convenient transport.
Sample should be collected in a sterile bottle CSBF, preferably with a pinch of sodium thiosulfate SBTD to neutralize residual chlorine, if any. Clean and sterile bottle with thiosulfate (SBTD), available from the IHS Laboratory, is suitable for collection of water samples for bacteriological analysis. In the absence of a sterile bottle with thiosulfate (SBTD), any clean and sterile bottle can be used. Health care institutions such as hospitals and diagnostic centres with in-house sterilisation facility may prepare their own sterile sample collection bottles.
If you must collect a sample and a sterile bottle is not available, a freshly emptied packaged drinking water bottle or a new PET bottle may be used, in that order.
Cation! Do not use empty beverage bottles such as ThumsUp, Maaza, Sprite, Coca Cola etc. Nutrients and other residues in such bottles may promote growth of bacteria and bias test results.
Step-2: Identify sampling point and time:
The best option is to identify a tap, spout or delivery pipe from which the sampling bottle can be filled. If there are many taps, identify a tap that is representative of the point-of-use. Remove tap-attachments, such as aerators and filters. For testing quality of municipal supply, select the direct municipal supply tap and choose a time during live municipal supply hours. To sample water from sumps, prefer pump-delivery either at the pump location or from inside the overhead tank (OHT) before water falls into the OHT. Similarly, prefer pump delivery pipes of borewells, and open wells. If taps and delivery pipes are not available and the water container has a narrow mouth, then pouring water into the sample collection bottle would be an option. But pouring option may not be available if the water storage container is so large that tipping it would not be feasible or it fixed as in case of a build overhead tank or underground sump. Thus, where tapping or pouring is not feasible a dip-sample would be the only option.
Step-3: Collect sample:
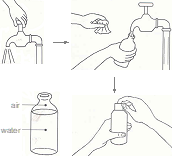
- Wash both your hands with soap and water, wipe with a clean towel and let it dry. Request an assistant to wash his/her hands and standby.
- Label the sample collection bottles and place it within easy reach, but do not open at this stage. Have ice packs ready.
- Then flush the tap, spout or delivery pipe, by letting water out for, say 2-3 minutes. Do not touch the tap or water flowing ou or t. In case of narrow mouth container, ask the assistant to tilt the container and pour water to flush its mouth. In case of dip sample, create a clear zone by carefully placing bottom of sample bottle on water surface and moving it in a cyclical to motion to disperse floating debris, if any.
- Collect the sample for physical and chemical analysis first; followed by collection of bacteriological-sample.
- Hold the sample collection bottle and wait for flushing of tap, spout, delivery pipe or container‑mouth is complete. Hold the bottle in one hand and open and hold the cap in the other hand avoiding to touch inner side of the cap. Place opened bottle mouth under the tap, delivery pipe, spout or mouth of the container avoiding direct contact with the tap. Ask the assistant to open the tap, delivery pipe lever or tilt the container to pour water into the bottle. As the bottle is about to be full, quickly remove it away from water stream and replace cap tightly. Wipe outside of bottle dry with a clean and dry tissue or cloth.
- Place each bottle inside separate dark‑colour bags, tie ice packs around each of them and place inside a carry bag for transport to laboratory.
Step-4: Transport to laboratory:
Transport the samples to laboratory as soon as possible, preferably within six hours. If you have multiple errands in the same trip, plan to first deposit sample the laboratory and then continue with other activities.
Step-5 Store sample, if required:
If immediate transport is not feasible, store the sample inside the regular chamber (not the freezer compartment) of a refrigerator until you are ready to transport it to the Laboratory, and definitely within 24 hours from the time of collection.
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the source of water, sampling point, activities & environment around the sampling point, details of how the sampling point is linked to the source, recent maintenance event (servicing of water purifier, cleaning of overhead tank, sump, repair and renovation of plumbing, etc.) if any. Mention the reason why you are ordering the test, as well as doubts and concerns, if any. This helps interpretation of test results. Occasionally, the IHS Laboratory may contact you for clarifications and additional information about the source and its environment, to help interpretation of test results.
Test Method & Duration:
The IHS Laboratory uses either membrane filtration and chromogenic-agar plating, as per IS 15185, 2016 RA 2021; OR enzyme substrate autoanalysis (ESA) for detection of coliforms & E. coli, as per APHA Standard Methods 24th Ed. 9223 B. Enzyme Substrate Test, 4.a Presence-absence (P/A) procedure. Both tests use 100 ml sample and are equivalent in terms detection accuracy. Result will be available after about 36 hours.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- IS10500, 2012. Table-6 in Indian Standard Drinking Water Specification. 2nd Revision. New Delhi: Bureau of Indian Standard (BIS); 2012 May, RA2023Amd4.
- WHO, 2022. Table 7.9, page 161 in Guidelines for drinking-water quality. 4th Edition, 1st & 2nd addenda. Geneva: WHO, 2022. https://www.who.int/publications/i/item/9789240045064.
- IS1622. 1981. Indian Standard Methods of Sampling and Microbiological Examination of Water. New Delhi: Bureau of Indian Standard (BIS); Indian Standard, IS1622 - 1981, Reaffirmed 2019.
- APHA Standard Methods 24th ed. 2023. American Public Health Association (APHA). Lipps WC, Braun-Howland EB, Baxter TE, eds. Standard Methods for the Examination of Water and Wastewater. 24th ed. Washington DC: APHA Press; 2023. https://www.standardmethods.org/about/.
- IS 15185, 2016 RA 2021 / ISO 9308-1, 2014. Water Quality - Detection and Enumeration of Escherichia coli and Coliform Bacteria - Membrane Filtration Method for Water with Low Bacterial Background Flora (1st Rev.). New Delhi: Bureau of Indian Standards (BIS).
