Bacterial Endotoxin Test (BET)
Scope:

Bacteriological Parameters:
Bacterial Endotoxin Test (BET) also known as Limulus Amoebocyte Lysate (LAL) assay.
Rationale:
Pyrogens are fever causing agents and endotoxin is a type of pyrogen. Endotoxin, is a water soluble, heat-stable fat-sugar (lipopolysaccharides) complex present in outer membrane of Gram-negative bacteria such as E. coli. These lipopolysaccharides (LPS) are a major component of Gram-negative bacterial cell wall and is essential for their survival. The release of LPS from bacteria takes place after death and lysis of the cell and is poisonous. A poisonous substance released by a living bacterial cell into its surroundings is called an exotoxin. On the other hand, a poisonous substance present in a living bacterial cell but released mostly upon its death and lysis is called an endotoxin. Thus, the fat-sugar complex released upon lysis of Gram-negative bacteria is called endotoxin. Endotoxin is commonly found everywhere in our environment. Neither boiling nor ordinary filtration can remove endotoxins.
As colonies of Gram-negative bacteria die large amounts of endotoxin is released into the environment and finds its way into water. As our intestinal flora contains Gram-negative bacteria, endotoxins are also produced inside our gut due to death of such colonies. But the intestinal mucosa prevents its passage into blood stream. If, somehow, endotoxins come in contact with blood, depending on the quantity, they elicit a range of body responses such as fever to more serious consequences like septic shock and death in extreme cases. The risk of endotoxins coming into contact with blood arises through injections into the bloodstream, exposure of blood through semipermeable membranes such as the case in haemodialysis, etc. The effect of endotoxin is related to its concentration and volume of contaminated liquid coming in contact with blood.
Water is one of the major commodities used by the pharmaceutical industry. Different grades of water quality are required for various pharmaceutical uses. These include, potable water, water for preparation of extracts, purified water and water for injection (WFI). Potable water may be adequate for synthesis of intermediates (bulk drugs) of active substances and manufacturing of chemical entities that do not require pyrogen free water. Pyrogens may not be an issue in case of water for preparation of certain extracts. Potable water may be adequate for cleaning and initial rinsing of pharmaceutical equipment and containers. However, the final rinse would require purified water or water for injection as is appropriate for various active substances. Most sterile medicinal products, including vaccines & parenteral preparations, require water for injection. Some sterile medicinal products like eye drops, ear, nose and skin preparations may use purified water. All non-sterile medicinal products must use purified water. Both WFI and Purified Water are bulk products, mostly used as raw material in pharmaceutical industry. The packaged water for injection product that is dispensed in retail pharmacies is actually named in the pharmacopeia as ‘sterile water for injection’. WFI must be pyrogen-free and sterile without any scope for regrowth of any microorganisms. That is why WFI systems are designed to maintain the product water at higher temperatures. Purified water is usually maintained at room temperature and may not be sterile as there is some scope for microbial regrowth. Tolerance of endotoxin, depends on intended purpose. For example, purified water for dialysis should be pyrogen free (EMA, 2018, USFDA, 1986).
The IS 17646 (Part 3) : 2021 / ISO 23500 part 3 : 2019, which gives requirements of water for haemodialysis and related therapies specify that the dialysis water should ideally be free of any pyrogen and the bacterial endotoxin level shall be < 0.25 EU/ml.
Traditionally, presence of endotoxin was tested using rabbit tests for pyrogens. In this method, the water to be tested is injected through a vein in to a rabbits' ear and temperature of the animal is observed according to a specified protocol. The rabbit pyrogen test required at least 3 rabbits, initial and half hourly recording of temperature of each rabbit for 3 hours and in case of indeterminate results, repeat test with 5 more rabbits. In addition to the long duration and cumbersome procedure, the rabbit pyrogen test has several other limitations. It is a pass/fail test, to determine whether the injected liquid has substantial pyrogen or not. The concentration of endotoxin could not be quantified.
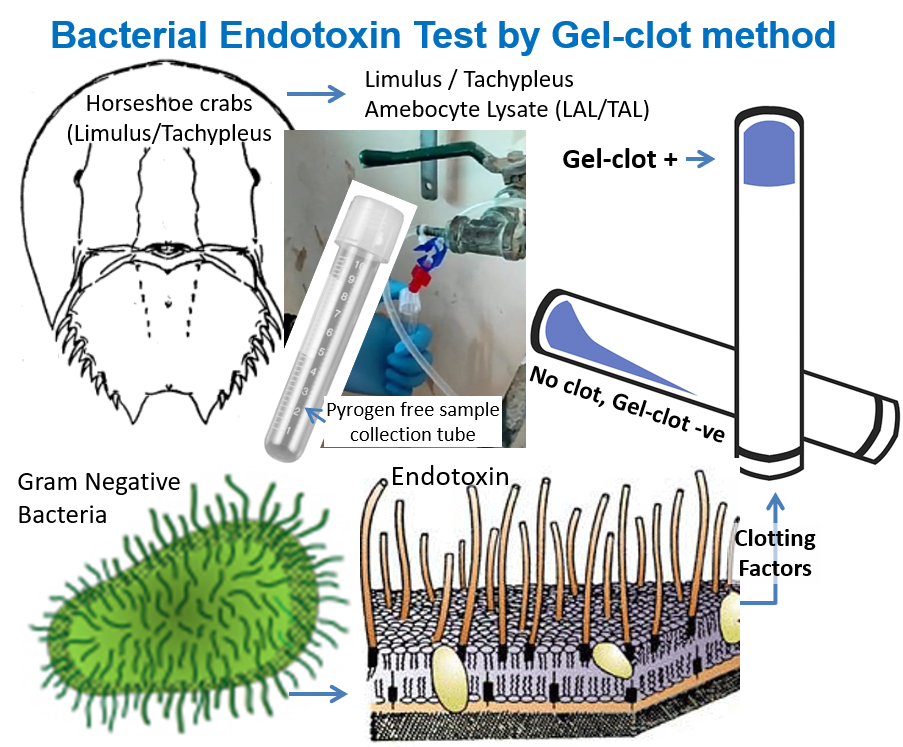
Discovery in 1960s, of the fact that bacterial endotoxin coagulates horseshoe crab (Limulus polyphemus) paved the way for development of more specific and quantitative bacterial endotoxin test, using Limulus amebocyte lysate (LAL) as a reagent (ERDG, 2018). There are two subfamilies of horseshoe crabs, namely; (a) the Limulinae, including Limulus polyphemus found in the east coast of North America, and (b) Tachypleinae found along the south & southeast east coasts of Asia from the Bay of Bengal to the South China sea. Both Tachypleus and Limulus Amebocyte Lysate (TAL and LAL, respectively) are used for bacterial endotoxin testing. There are four basic methods of endotoxin testing using the LAL or TAL reagent, namely; (i) the gel-clot; (ii) the turbidimetric (spectrophotometric); (iii) the colorimetric (Lowry protein); and (iv) the chromogenic assay.
The discovery of the horseshoe crabs’ immunological system, including the amoebocyte blood cell and the blood clotting factors contained within, changed the way products were tested for endotoxin. The blood of the horseshoe crab is blue due to the copper-based oxygen carrying protein hemocyanin. Frederik Bang and Jack Levin’s test uses the blood clotting system to form a gel clot in a test tube when endotoxin is present in the test sample. Research on horseshoe crabs showed that their blood is very sensitive to endotoxin, which is a component of Gram-negative bacteria like E. coli. In the 1960s (see timeline), Frederik Bang and Jack Levin developed a test from Limulus polyphemus blood that detected the presence of endotoxin. This test, based on the fact that the blood of the horseshoe crab gels or clots when it comes in contact with endotoxin, was called the Limulus amebocyte lysate (LAL) test and was commercialized in the United States in the 1970s. In Asia, there is a similar test called TAL which takes its name from an Asian species of crab, namely; Tachypleus tridentatus.
The United States Food and Drug Administration (USFDA) has recognized the benefits of the Limulus Amebocyte Lysate (LAL) based Bacterial Endotoxins Test (BET), particularly with respect to sensitivity, reproducibility, scope and simplicity. In 1984, five United States Pharmacopeia (USP) water products were given specific bacterial endotoxin limits. Water for Injection, Sterile Water for Injection and Sterile Water for Irrigation have an allowable endotoxin limit of 0.25 Endotoxin Units (EU)/ml. (EU=Unit of measurement for endotoxin activity). However, Bacteriostatic Water for Injection and Sterile Water for Inhalation have been given a slightly higher bacterial endotoxin limit of 0.5 EU/ml (USFDA, 1985). The Indian Pharmacopeia recognises BET as a valid method to test pyrogen-free status of pharmaceutical products.
The IHS Laboratory follows the gel-clot method for bacterial endotoxin test (BET) using LAL/TAL reagents.
Sample - Collection, Storage & Transportation:
Refer methods of sampling specified in IS 1622 : 1981 for bacteriological tests. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather Non-pyrogenic tube: You will need a sterile non-pyrogenic (endotoxin free) tube (NPT), available from IHS Laboratory.
Step-2: Identify sampling point and time:
Samples should be collected where a dialysis machine connects to the water distribution loop, and from a sample point in the distal segment of the loop or where such water enters a mixing tank. Hence, identify a convenient tap from the dialysis water distribution line, inside the dialysis room. As access to dialysis room is usually restricted, seek permission and follow protocol for entry into the dialysis room. Alternatively request a dialysis nurse or other health worker to help collect samples from any of the available dialysis water distribution taps from inside the dialysis room. Choose a day when the laboratory is open and collect in the forenoon, so that the sample can reach the laboratory by noon and processing done on the same day.
Step-3: Collect sample:
Before entering the dialysis area, wash both your hands with soap and water, wipe with a clean towel and let it dry. Put on disposable shoe covers and sterile gloves. Label the sample collection tube, but do not open at this stage. Have ice packs ready. Flush the delivery tap by letting water out into a bucket for, say 2-3 minutes. Do not touch the flow from delivery pipe. Grab the tube in one hand, open and hold the cap in the other hand avoiding to touch inner side of the cap. Place opened tube mouth under the tap avoiding direct contact. As the tube is about to be full, quickly remove it away from water stream and replace cap tightly. Wrap a dark‑colour sterile polythene (you can use a sterile glove) around the tube, and tie along with ice packs place a carry bag for transport to laboratory.
Step-4: Rush to laboratory as soon as possible. Do not store!
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the sampling point. Mention past tests or date of last sample collection. Gather details regarding source of feed water, simultaneous DRF & DLB samples if any, last date of disinfection of dialysis water distribution system, and the type of dialysis water distribution system.
Two types, namely direct and indirect feed dialysis water distribution systems are prevalent. In the direct feed water distribution systems, RO plant output passes through an endotoxin filter and is directly connected to the distribution loop delivering purified water to the various points of use in the dialysis room. Unused purified water is returned as feed water and is recycled through the RO plant. In indirect water distribution systems, the purified water from the RO plant is stored in a specially designed holding tank equipped with water‑level control devices. These devices interact with the RO plant, turning it off and on as needed and keeping the appropriate water level in the holding tank, so that the tank does not go dry or overfill. The purified water in the holding tank is repressurized by the distribution booster pump, which directs the purified water from the tank through an endotoxin filter before flowing out to the distribution loop, providing purified water to the various points of use in the dialysis room. Indirect purified water distribution systems return unused purified water back to the holding tank (Kasparek and Rodriguez, 2015).
Test Method & Duration:
Report will be available in 1 day.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- USFDA. Bacterial Endotoxins / Pyrogens. Silver Spring, MD, USA: United States Food & Drug Administration (USFDA); 1985 Mar 20; Inspection Technical Guides (ITG):40. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/bacterial-endotoxinspyrogens
- ERDG. 2018. Endotoxin Timeline. Little Creek, Dover, DE, USA: Ecological Research & Development Group (ERDG) Webpage: https://www.horseshoecrab.org/med/timeline.html accessed in April 2022.
- IS 1622: 1981. Indian Standard Methods of Sampling and Microbiological Examination of Water. New Delhi: Bureau of Indian Standard (BIS); Indian Standard, IS1622 - 1981, Reaffirmed 1996, Amended 2003. https://law.resource.org/pub/in/bis/S02/is.1622.1981.pdf
- IS 17646 (Part 3) : 2021 Preparation and quality management of fluids for haemodialysis and related therapies Part 3 Water for haemodialysis and related therapies. Identical under dual numbering (ISO 23500-3 : 2019).
- ISO 23500-3. 2019. Preparation and quality management of fluids for haemodialysis and related therapies — Part 3: Water for haemodialysis and related therapies. Geneva, Switzerland: International Standards Organization (ISO); 2019 Feb; ISO 23500-3:2019.
- Kasparek Ted and Rodriguez Oscar E. What Medical Directors Need to Know about Dialysis Facility Water Management. Clinical Journal of the American Society of Nephrology. 2015 Jun 5; 10(6):1061-1071.https://pubmed.ncbi.nlm.nih.gov/25979976/
- USFDA. Water for Pharmaceutical Use. Silver Spring, MD, USA: United States Food & Drug Administration (USFDA); 1986 Dec 31; Inspection Technical Guides (ITG), (ITG Subject: Water for Pharmaceutical use). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/water-pharmacuetical-use
- EMA. Guideline on the quality of water for pharmaceutical use. Draft. London, UK: European Medicines Agency (EMA); 2018 Nov 13; EMA/CHMP/CVMP/QWP/496873/2018. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-water-pharmaceutical-use_en.pdf
